Is it legal to eat insects in the UK?
Legality of edible insects in the U.K. – 2024 update
We’ve now received the official word straight from the Regulated Product Team at the FSA; this shouldn’t come as a surprise; as expected, all but three edible insect species are provisionally allowed to remain on the UK market: Acheta Domesticus (House crickets), Tenebrio Molitor Larvae (Mealworms), Gryllodes Sigillatus (Banded Crickets) until the regulator decides in favour or against commercialisation.
It’s interesting to note that, using heaven knows which safety criteria, the FSA has decided to allow Mealworms and Banded Crickets to remain on the market, even though no valid application exists; we are told that only once validated, Novel Food applications are included in this list [link here], and mealworms are banded crickets are not on it!
The aftermath of Brexit has sparked a regulatory rollercoaster, leaving entrepreneurs, investors, and insect enthusiasts questioning the legality of consuming these unique protein sources. In this post, we delve into the complex and evolving landscape surrounding edible insects, exploring the struggles faced by businesses and the perplexing regulations [Food and Feed (Miscellaneous Amendments) Regulations 2022].
A Rocky Road to Regulation
In July 2022, the Food Standards Agency made a significant admission, acknowledging a “mistake” in banning edible insects at the end of the Brexit transition period. We remain convinced that this “mistake” was influenced by an anti-edible insect sentiment prevalent in the political sphere or perhaps the meat lobbies.
Some mysterious forces compelled the esteemed FSA to finally admit its “mistake” after a mere two year delay and after the BBC began looking into the matter. A 10-year-old, armed with nothing but a firm grasp of the English language, could have read and understood how illogical FSA’s interpretation of the regulations was. Yet, these managed to confound our esteemed regulators for a very long time.
Perhaps only a public inquiry, conducted with utmost gravity, can unearth the unfathomable reasons behind the persistent refusal to acknowledge the “mistake”.
The FSA eventually was forced to recognise the need for transitional measures, and proposed a consultation that would allow edible insects to return to the market until December 2023. These measures were later enshrined in law in October 2022, providing a temporary reprieve for the industry.
More legislative ambiguity – Validation vs Approval
However, the Food and Feed (Miscellaneous Amendments) Regulations 2022 introduced a new legal ambiguity. When an application for an insect species is received by the FSA, the specific insect can remain on the market until “the appropriate authority informs the applicant, in accordance with Article 6(5) of Commission Implementing Regulation (EU) 2017/2469(3), that the application is not considered valid” or the process is terminated, either because the applicant or the authority terminates it.
Herein the confusion – a valid application does not equate or guarantee approval ! Yet some are under the impression that approval has already been granted.
Far from this, the journey from validation to approval is arduous, time-consuming, and costly, raising concerns for investors and lenders. This is exemplified by the Belgian Insect Industry Association’s [BiiF] application for House Cricket, which British companies were hoping to use as a basis for their dossiers. The BiiF application has been under review by the EFSA since July 3rd, 2018, as the EFSA continues to demand additional supporting data (with the most recent request made on March 23rd 2023). A clock stop/start mechanism within the law (both EU and UK), makes it possible to prolong the approval process pretty much indefinitely, provided the authority requests more and more scientific data or clarifications. 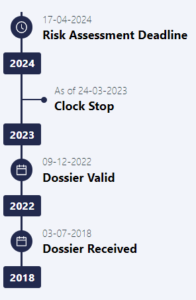
The lengthy approval process raises concerns about the projected timeframe for the FSA/FSS, which have comparatively less resources than the EFSA.
The lack of transparency surrounding the Novel Food assessment process further exacerbates the challenges faced by businesses seeking loans or investments.
Currently [June 2023] the FSA has ‘validated’ (not approved !) a dossier for crickets. We also expect that a Welsh company which received 2 millions from the public purse to produce an insect mince, might have also submitted a proprietary dossier to the FSA.
A tale of two approaches
A stark contrast emerges when comparing the European Food Safety Authority (EFSA) and the Food Standards Agency (FSA) in their handling of edible insect regulation. While the EFSA provides comprehensive information on received applications, including detailed summaries, applicant information, timelines, and meeting minutes, the FSA has chosen to withhold this data. We are told that only when a dossier is ‘validated’ the FSA will publish information the law dictates.
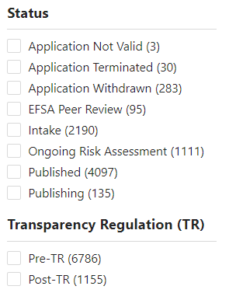




However we already can see an example of a ‘validated’ application, where the FSA continues to withhold information which by law should be published.
The lack of transparency from the FSA has raised questions about its obligations and the fairness of the approval process.
Incidentally the FSA has also rejected a request via the Freedom of Information Commissioner, concerning withholding the above data, appealing to Section 22 of the Freedom of Information Act (intention to publish the data at a later date).
The deadline looms
December 2023 acts as the deadline for companies to submit their novel food authorization to the FSA. Each insect species and processing method requires a separate evaluation, necessitating a substantial amount of scientific data. However, the timescale poses a significant challenge.
In any case, after validation, there is no guarantee that the process will conclude with a favourable opinion from the authority.
This has unsurprisingly left investors and lenders confused. The prevailing uncertainty within the regulatory landscape presents significant challenges for businesses seeking loans or attempting to attract investors, particularly those who have been provided with comprehensive information and a clear understanding of the situation.
The survival of the SMEs
The financial burden associated with obtaining novel food authorization poses a significant obstacle for UK companies. With costs estimated at £80,000-£100,000 per authorization, coupled with a 5-year+ timeframe (see BiiF example above), many small and medium-sized enterprises face an uncertain future. Unlike other countries such as Australia and New Zealand, which rely on independent assessments by their Food Standards Authority, the UK has chosen to burden companies with a complex and costly approval process.
Other competent bodies like the Uganda National Bureau of Standards, have also carried out scientific research, and defined microbiological criteria and safety standards for edible insects.
In other words, the FSA has not put any effort into establishing best practice guidelines, microbiological criteria etc. Instead it relies on approval and reapproval of the same product over and over again, for each individual company. This is irrespective of scientific evidence already in the public domain, as published in comprehensive EFSA Scientific Journals.
Can I eat insects in the UK ?
We understand the list of edible insects which could be sold in the U.K. until December 2023 was:
- Lesser mealworm (Alphitobius diaperinus larvae)
- House cricket (Acheta domesticus)
- Yellow mealworm (Tenebrio Molitor)
- Banded or decorated cricket (Gyllodes sigallatus)
- Desert locust (Schistocerca gregaria)
- Migratory locust (Locusta migratoria)
- Black soldier fly (Hermetia illucens larvae)
From the 1st January 2024, only species for which the FSA has received a valid application for, will be provisionally allowed to remain on the market, until the FSA rules in favour or against commercialisation. Because of the secrecy enshrining which edible insects are currently the subject of a “validated” application, and the lack of information concerning public versus data protected dossier, it is difficult to guess which insect will still be allowed and for which company.
Every farmer and manufacturer, both current and future, must continue to apply for authorisation. This is because, since edible insects are “novel food”, the method of preparation and the quantity in each product will require a separate evaluation and a separate set of scientific data.
A shift in power
The classification of insects as novel food raises questions about the motives behind this regulatory framework. Treating edible insects as novel food seems to favour capital venture-backed giants, allowing them to monopolise the European and UK markets. The government’s approach inadvertently or intentionally favours large corporations at the expense of SMEs, potentially altering the landscape of the industry.
Thanks to the classification of insects as Novel Food and the data protection provision within the regulation [(EU) 2015/2283 26-27], these large companies have obtained exclusivity in Europe and are set to take over the U.K. market.
If a company cannot afford to go through the Novel food authorisation, in order to survive, it has no option but to enter a ‘commercial agreement’ and become a ‘commercial partner’ of these capital venture giants.
Lastly, the only other companies which will benefit from the classification of edible insects as Novel Food, are those providing Novel Food consultancy services. Unsurprisingly these are the same players which advised the EU to follow the Novel Food route in the first place !
Take a look at some examples of duplicates
- Novel Foods – EFSA-Q-2021-00262 Request for a scientific opinion on Acheta domesticus powder as a novel food (NF 2020/1860)
- Novel Foods – EFSA-Q-2020-00748 Request for a scientific opinion on Dried Acheta domesticus as a novel food (NF 2018/0623)
- Novel Foods – EFSA-Q-2018-00543 Request for a scientific opinion on Acheta domesticus as a novel food (NF 2018/0128)
- Novel Foods – EFSA-Q-2019-00121 Request for a scientific opinion on Whole and ground crickets (Acheta domesticus) as a novel food (NF 2018/0804)
- Novel Foods – EFSA-Q-2019-00589 Request for a scientific opinion on defatted whole cricket (Acheta domesticus) powder as a novel food (NF 2019/1227)
A bleak future – 2024
In conclusion, the future of edible insects in the UK remains bleak. Those who invested in the sector, including the taxpayers, have every right to be outraged, to say the least. See for example how millions of public funds were invested through Innovate UK.
Because of the length and the cost of the Novel Food approval process, only one of the many thousands edible insect species, will possibly be authorised in the UK in the next few years.
Many SMEs will not be able to afford the cost of an application. They have been forced into applying jointly with competitors and feed the greed of the many self proclaimed consultants who are profiting from the situation. Unfortunately, while seemingly economically sound, the strategy of joint applications introduces complications compromising the approval process. In the case of edible insects, breeders utilising different feeds, including agri-food waste, leads to variations in nutritional composition and substance bioaccumulation, rendering the approval process complex and most likely inexact.
Brexit could have been an opportunity to disentangle the industry from the ludicrous classification of edible insects as Novel Food. Instead of doing its homework, the FSA prefers burdening the industry with the cost and the uncertainty. Instead of spending the taxpayers money efficiently, it prefers spending time processing the same insect species over and over again.
For completeness we should also report that more recently, in February 2023, consultancy firm Deloitte was awarded by the FSA the tender for a ‘Novel Food Regulatory Framework Review’, which aims at eventually reforming the UK regulatory novel food framework. A summary of the recommendations is published here. The outcome on the Deloitte’s review does not go into the specific issues we’ve highlighted in this post.
